|
|


|
Isotope
Atoms of the same elementówith the same Atomic Numberóhaving
the same numbers of protons and electrons, but with a different number
of neutrons, and therefore different Mass Numbers.

All isotopes of magnesium have 12 protons (& electrons).
Some isotopes of magnesium have 12 neutrons,
some have 13 neutrons and some 14.
Atomic Symbol
an abbreviation used to indicate the mass number and
atomic number of an isotope
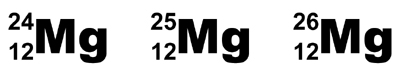
The Mass Number is written in the top left-hand
corner of the elemental symbol, with the Atomic Number written
below it, in the lower left-hand corner.
Isotopes of Magnesium
|
Atomic Symbol
|
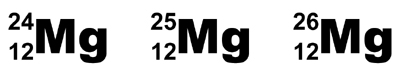
|
| # p+ |
12
|
12
|
12
|
| # e- |
12
|
12
|
12
|
| Mass Number |
24
|
25
|
26
|
| # nº |
12
|
13
|
14
|
| % Abundance |
78.99%
|
10.00%
|
11.01%
|
What are isotopes?
Practice Problems
Answer the following questions: |
|
1. State the number of protons
& neutrons in the following three isotopes of neon
(Ne).

2. Write an atomic symbol for each of the following
isotopes:
a) a
nitrogen atom with 8 neutrons
b) an
atom with 20 protons & 22 neutrons
c) an
atom with a mass # 27 & 14 neutrons
|
|
Atomic Mass
the weighted average mass of all the naturally occurring
isotopes of an element
Note that the Atomic Mass of Magnesium is closest to the
Mass Number of its most abundant isotope, Mg-24. |

|
Example:
78.99% of all magnesium isotopes is Mg-24:
.
0.7899 x 24.00 = 18.96
.
10.00% of all magnesium isotopes is Mg-25:
.
0.1000 x 25.00 = 2.500
.
11.01% of all magnesium isotopes is Mg-26:
.
0.1101 x 26.00 = + 2.863
(4 s.f.) 24.32
How is Atomic Mass calculated?
Practice Problems
Answer the following questions: |
|
1. If copper consists of two
isotopes, Cu-63 & Cu-65, are there more atoms of Cu-63
or Cu-65 in a sample of
copper?
Explain your answer.
2. Calculate the Atomic Weight of Copper if 69.2%
of all isotopes are Cu-63, and
30.8% are Cu-65.
|
|
|
|
| Sources: CHEMISTRY - an Introduction to General, Organic, &
Biological Chemistry, Prentice Hall CHEMISTRY, Modern CHEMISTRY,
CHEMISTRY
- the Central Science, and Principles & Applications of CHEMISTRY |
|
|


