Chemistry & Chemicals
-
What Is Chemistry and Why Is It Important?
Chemistry
The scientific study of the composition, structure,
properties and reactions of matter
Reactions are the processes of change matter undergoes,
and includes the energy changes that accompany these processes.
Matter
Anything that takes up space and has mass
Mass
The quantity of matter in any given object
Chemical
Any substance with a definite composition
Any solid, liquid or gas used in or produced by a chemical
process, either man-made or natural contains chemicals.
Chemicals—solids, liquids, or gases found in and
on the Earth, the crust of the Earth, the oceans and the atmosphere, living
organisms, comets, asteroids and the other planets of the solar system.
Things made of chemicals:
Air, gasoline, a dead leaf, a Snickers Bar, steel, wood,
shampoo, poop, urine, water, oxygen, gold, beer, etc.
-
Chemistry helps us understand our world:
Because living and nonliving things are made of matter
and matter is composed of chemicals, chemistry affects all aspects of life
and most natural processes.
What is chemistry and why is it
important?
Practice Problems
Answer the following questions: |
|
|
|
|
| 2. |
How do chemists define matter? |
|
|
| 3. |
What do we mean by mass? |
|
|
|
|
|
| 5. |
Which of the following are chemicals? |
|
.
| a) aluminum |
b) salt (sodium chloride) |
c) sugar (sucrose) |
| . |
|
|
| d) sunlight |
e) iron |
f) tin |
| . |
|
|
| g) a low temperature |
h) water |
i) air |
|
|
|
The Physical Properties & States
of Matter
-
What Are Physical Properties?
Physical Properties
Qualities or conditions of matter that can be observed
or measured without changing the composition of a substance
Physical properties can be classified as either extensive
or intensive.
Extensive Properties
physical properties that depend on the amount of matter
in a sample
Mass and volume are extensive properties.
Mass
the amount of matter an object contains |
Volume
the amount of space an object occupies |
Intensive Properties
physical properties that depend on the type of matter
in the sample, not the amount
Examples:
Melting Point
Boiling Point
State at 20 ºC (solid, liquid, gas)
Color
Luster (shine)
Odor |
Hardness
Malleability (can be molded into a shape)
Ductility (can be drawn into a wire)
Conductivity (of both heat and electricity)
Density |
-
Every pure substance has a unique set of intensive
physical properties.
Example:
|
Copper
|
Cu (cuprum) |
|
color:
|
reddish orange |
|
state at 20 °C:
|
solid |
|
odor:
|
odorless |
|
melting point:
|
1,083 °C |
|
boiling point:
|
2,567 °C |
|
luster:
|
very shiny |
|
conducts electricity:
|
excellent |
|
conducts heat:
|
excellent |
|
malleable
|
|
|
ductile
|
|
Copper is an element.
No other element except copper has this set of properties.
.
Substance (often used interchangeably with
“Chemical”)
Matter that has definite composition and properties
wherever it is found
-
Every sample of a given substance has identical intensive
properties because they all have the same composition
What are physical properties?
Practice Problems
Answer the following questions: |
|
| 6. |
What are physical properties? |
|
.
| a) Give four examples of a physical property. |
| . |
| b) Can two different elements have the same set
of physical properties? Explain. |
|
|
|
| 7. |
Distinguish between intensive and extensive
physical properties. |
|
| . |
Classify your four examples in #6 according to these
two types.
If you do not have at least two in each category, you
must add to your list. |
|
|
|
| 8. |
Distinguish between mass and volume. |
|
|
States of Matter
-
In what physical states does matter exist?
Solid,
Liquid,
and Gas
Solids
Definite shape and volume
Low-energy particles packed closely in a rigid arrangement
Not easily compressed
Tend to expand only slightly when heated
Example of a Solid:
iron
Liquids
Definite volume but indefinite shape
—takes the shape of its container
Mid-energy particles are close together, but free
to flow around one another
Not easily compressed
Tend to expand only slightly when heated
Example of a Liquid:
alcohol
Gases
Indefinite shape and volume
—takes the shape and fills the volume of its container
High-energy particles are relatively far apart and
move freely with little interaction
Easily compressed
Expand when heated
Example of a Gas:
carbon dioxide
Vapor
The gaseous state of a substance that is usually liquid
or solid at room temperature
Example:
Water vapor
Water is usually solid at room temperature, so when it
is in a gaseous state, water is referred to as a vapor.
Matter can also be transformed into a form called plasma:
Plasma
A form of matter in which the negatively charged particles
of an atom, the electrons, have been stripped away from the positively
charged nuclei. |
|
-
Unlike the three states of matter—solid, liquid, and gas—plasma
is not composed of chemicals.
Examples:
The sun and stars are composed of super-hot plasma,
some fires can burn hot enough to ionize the gases and
become plasma—such as burning magnesium.
In what physical states does
matter exist?
Practice Problems
Answer the following questions: |
|
| 9. |
What are the three states of matter? |
|
|
| 10. |
Which states of matter are being described? |
|
.
| a) Volume does not change in a different container |
| . |
| b) Has a very low density |
| . |
| c) Has large distances between particles |
| . |
| d) Shape depends on container |
| . |
| e) Particles have a fixed arrangement |
|
|
|
| 11. |
Which states of matter are being described? |
|
.
| a) Which state has a definite volume but takes
the shape of its container? |
| . |
| b) Which state takes on the volume and shape of
its container? |
| . |
| c) Which state has a definite volume and shape? |
|
|
|
| 12. |
Which is the technical difference between
a gas and a vapor? |
|
|
Change
Physical Changes
Changes affecting the form or appearance of a substance,
not its composition
-
Physical Changes can be classified as reversible
or irreversible.
Physical changes involving change of state are classified
as reversible.
Examples:
boiling, melting, freezing, condensation
Changes in the form of a solid are irreversible.
Examples:
breaking, splitting, cutting, grinding, crushing, cracking
-
How can matter be changed from one form to another?
Change of State
when matter is converted from one state to another
by adding or removing energy
Changes of State are reversible.
Example:
Freeze or boil water, or freeze it and then smash it
into a million pieces—it will still be water.
Melting Point & Freezing Point
going from solid to liquid or liquid to solid
melting point
melting—changing from a solid to a liquid
solid is heated
particles begin moving faster
until they gain sufficient energy
overcome attractive forces holding them together
The melting point of something is also the temp at which
it freezes.
| Water: |
(freezes/melts)
|
0°C
|
| Gold: |
|
1,064°C
|
| Nitrogen: |
|
-210°C
|
freezing point
freezing—changing from a liquid to a solid
temperature of liquid is lowered
energy in the particles of the liquid is lost
particles move slower
attractive forces pull particles close together
Sublimation & Deposition
going from solid to gas or gas to solid without going
through a liquid state
Sublimation
particles on surface of solid absorb enough heat to
change directly to gas with no temperature change and without going through
the liquid state
Example:
Dry ice, frozen CO2, sublimes at -78°C.
CO2 does not form a liquid.
Deposition
when gas changes directly to solid
Example:
Frost on windows in sub-freezing air
Evaporation
high energy particles at the surface of a liquid changing
to gas, leaving the remaining liquid cooler overall
-
When heat is removed, a reverse process takes place.
Condensation
the change of state from gas to liquid
Boiling Point
the temperature at which a liquid changes to gas (boils)
and gas changes to liquid (condenses)
Boiling
the formation of gas bubbles from the liquid
Heating and Cooling Curves
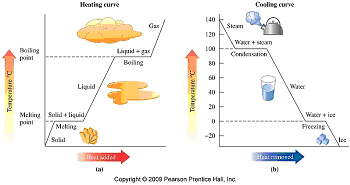
What is the difference between
reversible and irreversible changes of state?
Practice Problems
Answer the following questions: |
|
| 13. |
What is a physical change? |
|
|
|
|
| 14. |
What is the difference between a reversible
and an irreversible change?
Give an example of each. |
|
|
|
|
| 15. |
What is a change of state? |
|
|
|
|
| 16. |
List and define seven changes of state. |
|
|
|
|
| 17. |
What are the two phase changes that “skip
over” one of the states? |
|
|
|
|
| 18. |
How do you know when something is boiling? |
|
|
|
|
| 19. |
Using a cooling curve for water, identify
the state or change of state for water as solid, liquid,
gas, condensation or freezing. |
|
.
| a) at 120 °C |
b) at 100 °C |
c) at 40 °C |
| . |
|
|
| d) at 0 °C |
e) at –10 °C |
|
|
|
|
|


