Quantum Mechanics
The description of an electron and its behavior
Quantum Mechanical Model of
the Atom
Determines the allowed energies an electron can have
and how likely it is to find the electron in various locations around the
nucleus
Quantum
The amount of energy needed to move an electron from
one energy level to another
Ground State
The lowest or original energy level of an electron
Principle Quantum Number (n)
The number representing the energy level of the electron
-
Of the elements known today, there are seven energy
levels
(n = 1 to n = 7).
For each value of n there may be several orbitals
with different shapes and at different energy levels.
When the orbit of an electron is defined solely
by the Principle Quantum Number (n), the orbit is circular
(just as suggested by the Bohr Hydrogen Model).
Orbitals
The regions around the nucleus where electrons of
a certain energy are more likely to be found
As the number of the energy level, n, increases,
-
both the size of the orbital
-
and its energy level increases
Energy sublevels
energy levels within a principle energy level
Each energy sublevel corresponds to an orbital of a different
shape, which describes where the electron is likely to be found.
The second quantum number, called the Azimuthal
Quantum Number (l), defines the shape of the
orbital.
l can have values from 0 to n – 1
(l = 0 to l = n - 1).
There are four main types of orbitals—s,
p,
d,
& f (and even a fifth, g!).
| value of n |
1
|
2
|
3
|
4
|
5
|
value of l
(n - 1) |
0
|
1
|
2
|
3
|
4
|
| orbital |
s
|
p
|
d
|
f
|
g
|
-
Each orbital can hold a maximum of two electrons.
What is Quantum Mechanics?
Practice Problems
Answer the following questions: |
|
| 1. |
What is Quantum Mechanics? |
|
|
| 2. |
What is the Quantum Mechanical Model of
the Atom? |
|
|
|
|
|
| 4. |
What is the Ground State of an electron? |
|
|
| 5. |
What does the Principle Quantum Number
(n) indicate? |
|
. |
|
How many energy levels are known today? |
|
|
| 6. |
What are orbitals? |
|
. |
|
What are two things that happens to the electron
orbital as the number of the energy level, n, increases? |
|
|
| 7. |
What are Energy Sublevels? |
|
|
| 8. |
What does the Azimuthal Quantum Number
(l) indicate? |
|
|
| 9. |
What are the four main types of orbitals? |
|
|
| 10. |
What is the maximum number of electrons any
orbital can hold? |
|
|
Electron Orbitals & Electronic
Configuration
s orbitals
s orbitals are spherical, with the nucleus at
the center.
There is one s orbital in each energy level,
with two s electrons total. |
|
|
-
The elements in Group 1A (1) have one s orbital
electron.
-
The elements in Group 2A (2) – 8A (18) have two s
orbital electrons.
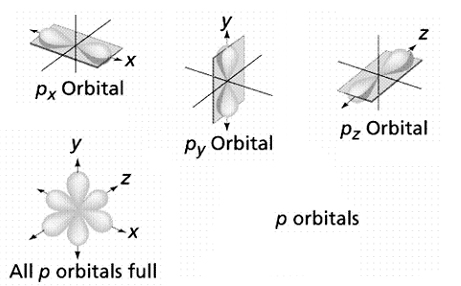
p orbitals
Starting with the second energy level (n = 2),
there is a set of three p orbitals in each
energy level.
Each orbital can contain up to two electrons
for a maximum of six p orbital electrons
in each energy level.
The p orbitals in each energy level are arranged
along the x, y, & z axes around the nucleus, respectively. |
|
 |
-
The elements in Group 3A (13) have one p orbital
electron.
-
The elements in Group 4A (14) have two p orbital
electrons, and so on, up to
-
The elements in Group 8A (18), which have six p
orbital electrons.
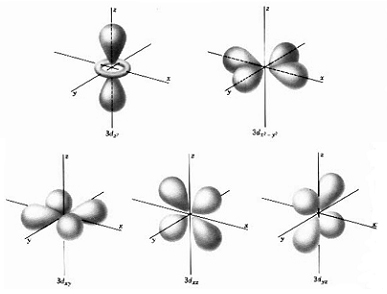
d orbitals
Starting with the third energy level (n = 3),
there are five d orbitals
with a maximum of two electrons in each orbital,
for a maximum of ten d orbital electrons
in each energy level.
-
The order in which the d orbital electrons are filled
cannot be determined by the periodic table alone, but must be experimentally
determined.
|
|
 |
f orbitals
Starting with the fourth energy level (n = 4),
there are seven f orbitals
with two electrons possible in each orbital,
for a maximum of 14 f orbital electrons
in each energy level.
-
The order in which the f orbital electrons are filled
cannot be determined by the periodic table alone, but must be experimentally
determined.
What is the maximum number of electrons
each orbital can hold?
Practice Problems
Answer the following questions: |
|
| 1. |
How many s orbitals does each
energy level have? |
|
What is the maximum number of s orbital
electrons for any value of n? |
|
. |
|
Can the order in which the s orbitals of an atom
are filled in any given energy level can be determined simply from its
position on the periodic table? |
|
|
| 2. |
What is the first energy level to
have p orbital electrons? |
|
. |
|
How many p orbitals can an energy level
have? |
|
What is the maximum number of electrons each p
orbital can hold? |
|
What is the total number of p electrons for any
value of n? |
|
. |
|
Can the order in which the p orbitals of
an atom are filled in any given energy level can be determined simply from
its position on the periodic table? |
|
|
| 3. |
What is the first energy level to
have d orbital electrons? |
|
. |
|
How many d orbitals can an energy level
have? |
|
What is the maximum number of electrons each d
orbital can hold? |
|
What is the total number of d electrons for any
value of n? |
|
. |
|
Can the order in which the d orbitals of
an atom are filled in any given energy level can be determined simply from
its position on the periodic table? |
|
|
| 4. |
What is the first energy level to
have f orbital electrons? |
|
. |
|
How many f orbitals can an energy level
have? |
|
What is the maximum number of electrons each f
orbital can hold? |
|
What is the total number of f electrons for any
value of n? |
|
. |
|
Can the order in which the f orbitals of
an atom are filled in any given energy level can be determined simply from
its position on the periodic table? |
|
|
|
Summary
of Principle Energy Levels, Sublevels, and Orbitals
|
|
|
|
|
|
|
n = 1
|
1
|
|
|
n = 2
|
2
|
|
2s (1 orbital), 2p (3 orbitals)
|
|
|
n = 3
|
3
|
3s (1 orbital), 3p (3 orbitals),
3d (5 orbitals)
|
|
|
n = 4
|
4
|
4s (1 orbital), 4p (3 orbitals),
4d (5 orbitals), 4f (7 orbitals)
|
|
| . |
. |
. |
Shells, Subshells, & Orbital
Filling
Electron Shell
All the orbitals within the same energy level
All the electrons would share
the same Principle Quantum Number (n =
1 to n = 7)
and Azimuthal Quantum Number (s, p,
d,
f).
Example:
2s2
2p6
All the s and p orbital electrons in the
second energy level
(n = 2) are in the second electron shell.
Subshell
All the electrons of one type of orbital in
the same energy level.
Example:
4d10
All the d orbital electrons in the fourth energy level
(n = 4) are in the 4d subshell.
Each shell is divided into the number of
subshells equal to the Principle Quantum Number, n, for that shell.
The first energy level (n = 1) has one subshell,
1s.
The second energy level (n = 2) has two subshells,
2s & 2p.
The second energy level (n = 3) has three subshells,
3s, 3p, & 3d, and so on...
Each subshell is divided into orbitals.
The division of orbitals in each energy level is the progression of odd
numbered integers, so that
there is 1 s orbital
there are 3 p orbitals
there are 5 d orbitals
and there are 7 f orbitals.
Magnetic Quantum Number
The Magnetic Quantum Number (ml)
can have integral values between l and –l, including zero.
This quantum number describes the orientation of the orbital in space.
Spin Quantum Number
(electron spin).
| The Electron Spin is the reason why it
takes two, but only two, electrons to fill an orbital. Once an orbital
has two electrons, the next electron must go on to the next orbital. |
|
What is the difference between a
shell and a subshell?
Practice Problems
Answer the following questions: |
|
| 1. |
What is an electron shell? |
|
|
| 2. |
What is the maximum number of electrons in
the second electron shell? |
|
|
| 3. |
What is an electron subshell? |
|
|
| 4. |
What is the maximum number of electrons in
the 3p subshell? |
|
|
| 5. |
What is the maximum number of electrons in
the 5d subshell? |
|
|
Writing Orbitals
In the atom, electrons and the nucleus interact to make
the most stable arrangement possible.
Electron Configurations
the way electrons are arranged in certain discrete
orbitals around the nucleus
-
Three rules—the Aufbau Principle, the Pauli
Exclusion Principle, and Hund’s Rule—describe how
to find the electron configurations of atoms.
Aufbau Principle
Electrons occupy the orbitals of lowest energy first
The p orbitals all have more energy than the s
orbitals of the same level.
Example:
The 2p orbital has more energy than the 2s
orbital,
the 3p orbital has more energy than the 3s,
and so on.
However, the d orbitals have slightly more energy
than the s orbitals of the next higher level,
and the f orbitals have slightly more energy than
the s orbitals of the next TWO higher levels.
Example:
The 3d orbital has slightly more energy than the
4s orbital (but not as much as the 4p orbital) and so on,
and
the 4f orbital has slightly more energy than the 6s orbital
(but not as much as the 6p orbital) and so on.
Pauli Exclusion Principle
Any orbital may contain only two electrons, and those
electrons must be of opposite spins—clockwise and counterclockwise.
Remember, that for any energy level (n):
-
s has one orbital—for a maximum of 2 electrons,
-
p has three orbitals—for a maximum of 6 electrons,
-
d has five orbitals—for a maximum of 10 electrons,
and
-
f has seven orbitals—for a maximum of 14 electrons.
Hund’s Rule
Electrons occupy orbitals of the same energy so as to
make the number of orbitals of the same spin direction as large as possible.
What are the three rules that
describe how to find the electron configurations of atoms?
Practice Problems
Answer the following questions: |
|
| 1. |
What would be the electron configuration
of sulfur, S? |
|
|
| 2. |
What would be the electron configuration
of arsenic, As? |
|
|
| 3. |
What would be the electron configuration
of lead, Pb? |
|
|
|


